
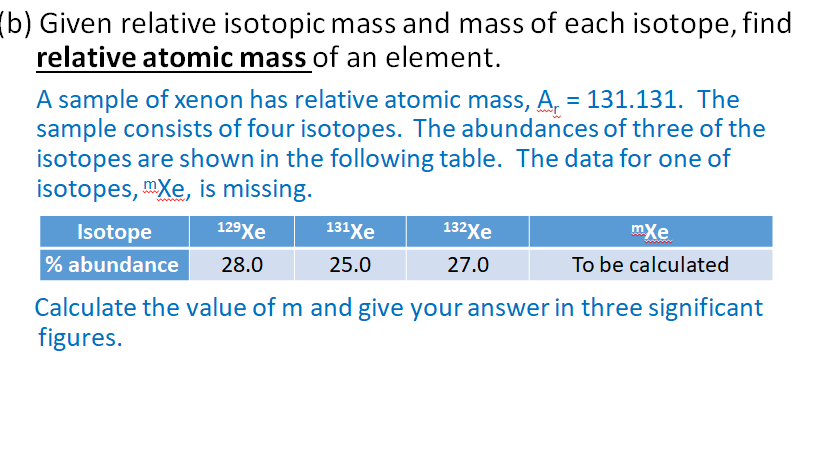
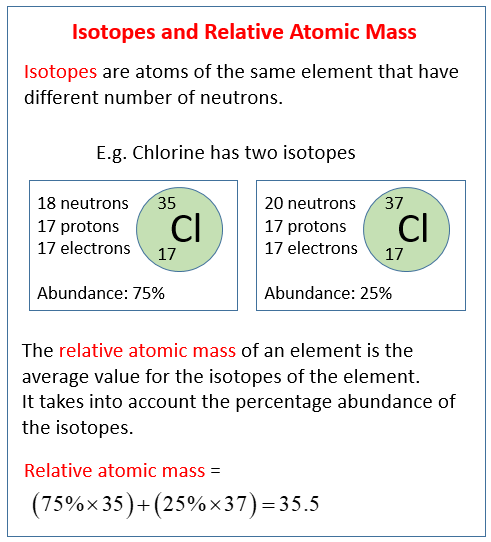
- Find out how isotopes can be detected using mass spectrometry. Learn about isotopes and how they relate to the average atomic mass of an element. Find out how isotopes can be detected using mass spectrometry. If you're seeing this message, it means we're.
- In the general chemistry series we learned about nuclide symbols, which all imply a specific atomic number and mass number. Associated with this were isotope.

Isotopic measurements can also provide input for mass-balance calculations and quantitative constraints on reaction progress. 2.2 Fundamentals of Isotope Geochemistry The following section presents a very brief discussion of the fundamentals of stable and radio-isotope geochemistry, intended to provide readers with the necessary background.
Isotopic Mass Definition
'Isotopes' | |
- | |
Monoisotopic and Average Mass | |
| - | |
| -- | |
| - | |
-Figure A. | Isotopes Figure A. shows a simulated isotopic distribution of the [M+H]+ ion of a compound with the following elemental composition, C48 H82 N16 O17 (Poly-Alanine) The Program IsoPro ver 3.1 was used for the simulation. The resolving power was set to 10,000. |
| . | . |
Monoistopic Mass = 1155.6 Average Mass = 1156.3 (calculated) As shown in Figure B. the monoisotoptic mass of this compound is 1155.6. For a given compound the monoisotopic mass is the mass of the isotopic peak whose elemental composition is composed of the most abundant isotopes of those elements. The monoisotopic mass can be calculated using the atomic masses of the isotopes. The average mass is the weighted average of the isotopic masses weighted by the isotopic abundances. The average mass can be calculated using the atomic weights of the elements. The calculated average mass is shown in Figure B with the red line. | Figure B. |
| . | . |
| . | . |
Figure C.- | Average mass can be measured by attempting to centroid a low resolution profile of a compound. The better the centroiding program the closer the measured mass will be to the theoretical average mass. The yellow envelope, shown in figure C., depicts how the peak might look at low resolution (perhaps with a low res. quadrupole instrument). A good centroiding program will be able to find the center of mass of this peak (yellow blob). A not so good program (that's a euphemism) will only pick the peak top. So with bad software, the reported mass for this compound would be closer to the monoisotopic mass. As the A+1 peak grows with increasing mass the peak top of the low resolution peak will inch closer to the theoretical average mass, and the program will begin to report values closer to the average mass. One might attempt to measure the masses and the abundances of the isotopes (at a higher resolution) then calculate the average mass of the compound but this exercise is seldom performed in routine analysis. |
| -- | |
| Once again we would like to reiterate that at lower resolution a peak labeling program might report masses closer to the monoisotopic mass when the compound mass is below 1500 amu and may report masses closer to the calculated average mass when the compound is larger than 1500 amu. | |
| - | |
Previous, Slide 7 | |
. | |
.(slide jumper) ContentsGlossaryComp.12345678 - | |
home | disclaimer | |
